Why Lithium Batteries Fear the Cold?
The fear of cold temperatures by lithium batteries is primarily related to their internal chemical reactions and physical properties.
1. **Slowing of Chemical Reaction Rates**
During the charging and discharging processes of lithium batteries, the migration of lithium ions between the cathode and anode, as well as the chemical reactions of the electrode active materials, are involved. These reactions require a certain amount of energy to overcome the activation energy barrier for electron transfer and material transformation.
In low-temperature environments, the rate of these chemical reactions significantly slows down because the molecular motion of the reactants decreases, leading to a reduction in collision frequency, making the reactions difficult to proceed. As a result, the performance of lithium batteries at low temperatures is affected, manifesting as reduced capacity and decreased rate performance.
2. **Decreased Solubility of Electrode Active Materials**
The solubility of the electrode active materials inside lithium batteries decreases at low temperatures. This means that under cold conditions, the concentration of electrode active materials in the electrolyte will decrease, thereby reducing the amount of active materials available for reactions. This further reduces the battery’s charging and discharging capabilities, leading to a decline in battery performance.
3. **Reduced Electrolyte Mobility**
The electrolyte in lithium batteries becomes viscous and can even solidify partially at low temperatures. This leads to a decrease in the ion conductivity of the electrolyte, i.e., the migration speed of ions in the electrolyte slows down. Since ion migration is a key part of the battery’s charging and discharging process, the reduced mobility of the electrolyte directly affects the battery’s output voltage and power output.
4. **Increase in Internal Resistance**
In low-temperature environments, the resistance of various materials inside the lithium battery (such as electrodes, electrolytes, etc.) increases. This leads to an increase in the battery’s internal resistance, thereby reducing the battery’s effective capacity and discharge time. At the same time, the increase in internal resistance also causes the battery to generate more heat during charging and discharging, further affecting the battery’s performance and safety.
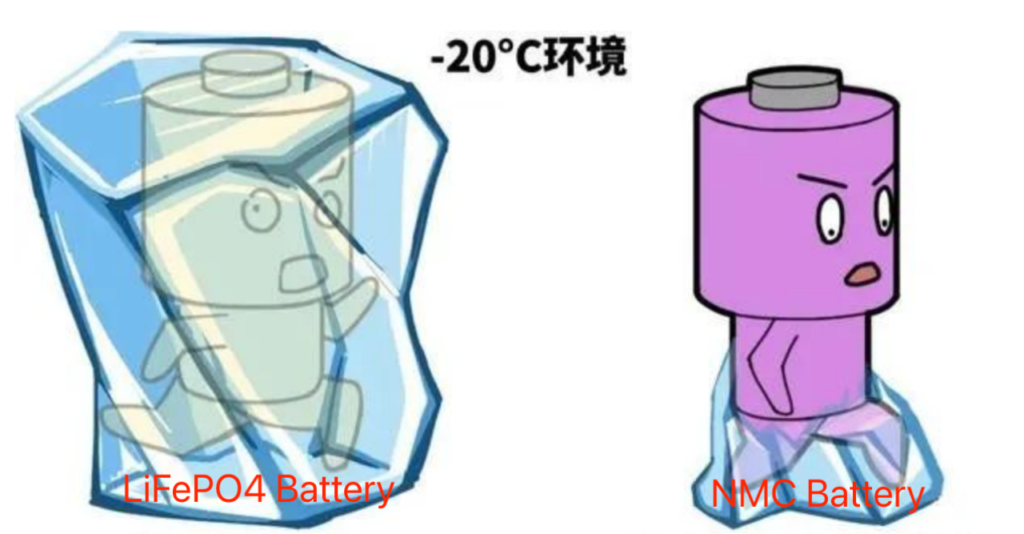
5. **Anode Lithium Plating Phenomenon**
During low-temperature charging, lithium ions are prone to form lithium dendrites on the surface of the anode. These lithium dendrites may puncture the separator, leading to internal short circuits and safety incidents. Additionally, the deposited metallic lithium reacts with the electrolyte to form deposits, increasing the thickness of the solid electrolyte interphase (SEI), which further affects the battery’s low-temperature performance.
6. **Solutions to Address Lithium Battery Aversion to Low Temperatures**
1. **Improvement of Battery Materials and Design**
Optimize the electrolyte: Use electrolytes that are not prone to freezing to ensure good ion transport capabilities at low temperatures. Improve electrode materials and battery structure design, reasonably design the internal structure of the battery to reduce internal voids and enhance the battery’s thermal conductivity.
2. **Battery Management System Optimization**
Intelligent temperature control system: Add temperature sensors inside the battery to monitor temperature changes in real-time.
Take heating or cooling measures based on battery temperature to ensure the battery maintains an appropriate working temperature in low-temperature environments.
Battery preheating technology:
Preheat the battery to an appropriate temperature before use to improve the low-temperature performance of lithium batteries.
This can be achieved through vehicle preheating systems or home charging pile preheating functions.
Adjust charging strategies:
Use slow charging methods in low-temperature environments to reduce the battery’s internal resistance and the rate of electrochemical reactions.
Protect the battery from overcharging and over-discharging by controlling the battery’s current and voltage.
3. **User Suggestions and Measures**
Avoid long-term low-temperature parking: Try to avoid parking vehicles in low-temperature environments for extended periods to reduce the rapid decline of battery power.
Charge as needed: Follow the principle of “charge as needed” to avoid low battery levels affecting normal use.
Choose high-quality insulation materials: Wrap lithium batteries with specialized insulating materials to increase their temperature.
Pay attention to purchasing high-quality insulating materials to ensure effective insulation.







