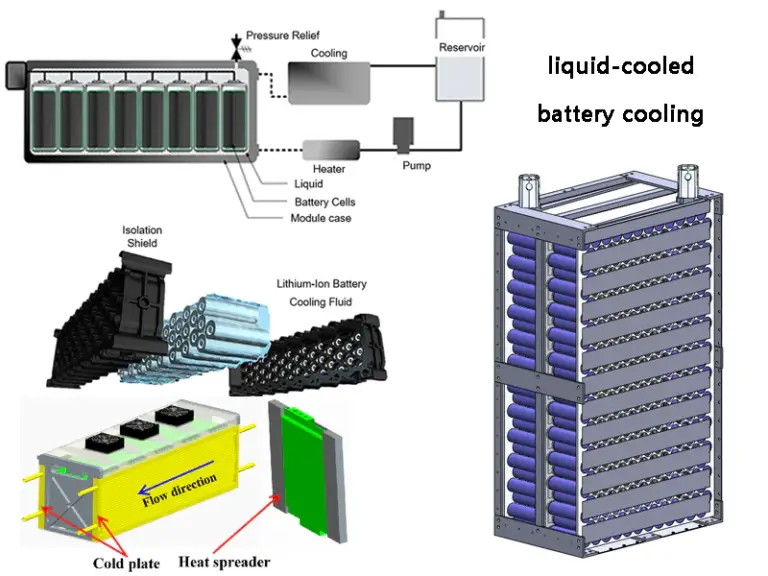
Lithium Battery Basic Knowledge
Introduction to Metallic Lithium
Lithium is a metallic element, positioned at the forefront of metals in the periodic table. Its chemical symbol is Li, and it is a silver-white, extremely soft, and highly reactive metal. It is the lightest metal among all metals, and in lithium batteries, it is typically used in the form of compounds as the positive electrode.
What is a Lithium-ion Battery?
Any chemical power source that uses compounds of metallic lithium as the positive electrode is collectively referred to as a lithium-ion battery. Rechargeable lithium-ion batteries are known as secondary batteries (also known as storage batteries). They can convert electrical energy into chemical energy for storage and then convert it back into electrical energy when in use, which is a reversible process.
Basic Concepts of Lithium Batteries
Voltage (V): Refers to the potential difference between two points in a circuit, which enables the flow of electrons.
Open-circuit voltage: Refers to the voltage of a battery when it is not connected to an external circuit or load. The open-circuit voltage has a certain relationship with the remaining energy of the battery, and the SOC display utilizes this principle.
Operating voltage: Also known as terminal voltage, it refers to the potential difference between the positive and negative poles of the battery when it is in a working state, i.e., when there is current flowing through the circuit. When the battery is working in a discharge state, the operating voltage is always lower than the open-circuit voltage because the current must overcome the internal resistance. The opposite is true during charging.
Cut-off discharge voltage: Refers to the voltage reached when a fully charged battery is discharged until it is depleted (if continued discharging occurs, it is considered over-discharging, which can damage the battery’s life and performance).
Charge limit voltage: The voltage value at which the charging process transitions from constant current to constant voltage.
Internal resistance (mΩ): Refers to the resistance encountered by the current when it flows through the interior of the battery during operation.
The size of the internal resistance is mainly affected by the battery’s materials, manufacturing processes, and structure.
The internal resistance of a battery includes ohmic resistance and polarization resistance. Ohmic resistance is composed of the resistance of electrode materials, electrolytes, separator, and contact resistance of various parts, while polarization resistance includes resistance caused by electrochemical polarization and concentration polarization.
The internal resistance of a battery is a very complex and important characteristic. Factors affecting internal resistance include: ① main materials; ② structure.
Due to the existence of internal resistance, when the battery discharges, the current passing through the internal resistance generates heat and consumes energy. The greater the current, the more energy is consumed. Therefore, the smaller the internal resistance, the better the performance of the battery. Not only is the actual working voltage of the battery higher, but the energy consumed on the internal resistance is also less.
Battery capacity (Ah): Refers to the amount of electric charge obtained from the battery or the amount of electric charge the battery can store.
Capacity is an important indicator of battery performance, determined by the capacity of the electrodes. If the capacities of the electrodes are not equal, the capacity of the battery depends on the electrode with the smaller capacity, not the sum of the capacities of the positive and negative poles.
Capacity is represented by C and is measured in Ah (ampere-hours) or mAh (milliampere-hours).
Formula: C=It, which means battery capacity (Ah) = current (A) × discharge time (h).
A battery with a capacity of 10 ampere-hours can discharge for 2 hours at 5 amperes and for 1 hour at 10 amperes. C2 represents the capacity of the battery measured after discharging for 2 hours, and C5 represents the capacity measured after discharging for 5 hours.
The actual capacity of a battery mainly depends on the following factors: the quantity and quality of active materials; and the utilization rate of active materials.
Battery energy (Wh): Refers to the amount of electric charge (energy) stored in the battery, represented by Wh.
Formula: Energy (Wh) = Rated voltage (V) × Capacity (Ah).
Significance: A single cell with a voltage of 3.7V and a capacity of 15Ah has an energy of 55.5Wh, and a battery pack with a voltage of 37V and a capacity of 10Ah has an energy of 370Wh. Battery energy is an important indicator for measuring the work done by the battery in driving devices; capacity alone cannot determine the amount of work done.
Energy density (Wh/Kg): Refers to the energy released per unit volume or mass, usually expressed as volumetric energy density (Wh/L) or specific energy density (Wh/kg).
For example, a lithium battery weighing 325g, with a rated voltage of 3.7V and a capacity of 10Ah, has an energy density of 113Wh/kg. The following table shows theoretical values, and in practical applications, factors such as the casing and parts in the battery structure must be considered. Currently, the energy density of lithium batteries is 3 times that of nickel-cadmium and 1.5 times that of nickel-metal hydride batteries. The level of energy density is determined by the material density and structure.
Discharge rate (A): The discharge rate refers to the current value required to discharge its rated capacity (C) within a specified time (N), which is numerically equal to the multiple of the battery’s rated capacity.
The discharge rate and discharge rate are reciprocals: 1/N rate discharge = N-hour rate discharge; that is, 1/N*C=C/N.
Taking a 10Ah battery as an example:
5-hour discharge rate = C/5 = 10Ah/5h = 2A = 0.2C
0.5-hour discharge rate = C/0.5 = 10Ah/0.5h = 20A = 2C
Charging methods: CC/CV: CC means constant current, charging the battery with a fixed current; CV means constant voltage, charging the battery with a fixed voltage, and the charging current will decrease as the voltage is reached.
Trickle charging: Refers to charging the battery with a current less than 0.1C. It is generally used for supplementary charging when the battery is close to full. If the load does not have strict requirements for charging time, it is recommended to use the trickle charging method (in this case, the battery life is longer).
Charge and discharge depth (COD DOD): The method of expressing the remaining capacity value of the battery, the charge and discharge depth are represented as a percentage rate. For example, a battery with a capacity of 10Ah, after discharging, the capacity becomes 2Ah, which can be called 80% DOD; a battery with a capacity of 10Ah, after charging, the capacity is 8Ah, 80% COD. Full charge and discharge are usually referred to as 100% DOD.
The charging status is called SOC: A 10Ah battery, when in a 5Ah state, becomes 50% SOC.
Self-discharge rate (%/month):
Definition: During the storage (open circuit) process, the battery’s capacity will gradually decrease. The proportion of the reduced electricity to the capacity is called the self-discharge rate.
Causes: Due to the instability of the electrodes in the electrolyte, chemical reactions occur at the two electrodes of the battery. Active materials are consumed, and the chemical energy converted into electrical energy is reduced, leading to a decrease in battery capacity.
Influencing factors: The ambient temperature has a significant impact, and high temperatures will accelerate the self-discharge of the battery.
Expression: The expression method and unit for battery capacity decay (self-discharge rate) are: %/month.
Results: Battery self-discharge will directly reduce the battery’s capacity. The self-discharge rate directly affects the storage performance of the battery. The lower the self-discharge rate, the better the storage performance.
Cycle life (times):
Concept: A secondary battery that undergoes one charge and discharge is called a cycle or one cycle. After repeated charge and discharge, the battery’s capacity will gradually decrease. The number of cycles the battery undergoes before the electric capacity drops to a specified value under certain discharge conditions is the cycle life.
Definition: When the battery capacity drops to 80%, the number of charge and discharge times is called the cycle life.
Influencing factors: Incorrect use of the battery, battery materials, the composition and concentration of the electrolyte, charge and discharge rates, discharge depth (DOD%), temperature, manufacturing process, etc., all affect the cycle life of the battery.
Discharge platform: Refers to the part of the discharge curve where the voltage remains basically flat. The longer and more stable the discharge platform, the better the discharge performance of the battery.
Battery pack consistency: A battery pack is composed of multiple single cells connected in series and parallel. The overall performance and life of the battery pack depend on the cell with the poorest performance, which requires a high degree of consistency in the performance of each cell in the battery pack. In addition to the errors in the performance of individual cells and the quality of raw materials, the main reason is the manufacturing process. Improvements in the process are very important for improving the quality of the battery.
Formation: After the battery is made, the process of activating the internal positive and negative electrode active materials through a certain charge and discharge method, improving the battery’s charge and discharge performance, and comprehensive performance such as self-discharge and storage, is called formation. The battery can only reflect its true performance after formation. At the same time, the sorting process during the formation can improve the consistency of the battery pack, thereby enhancing the performance of the final battery pack.