100 Questions about Batteries
Basic Principles and Terminology of Batteries
1.What is a battery?
A battery is a device for energy conversion and storage that converts chemical energy or physical energy into electrical energy through reactions. Based on the energy conversion, batteries can be classified into chemical batteries and physical batteries. Chemical batteries or cells convert chemical energy into electrical energy. They consist of two different components: electrochemical active electrodes for positive and negative terminals, and a chemical substance that serves as an electrolyte to facilitate conduction. When connected to an external circuit, they convert internal chemical energy into electrical energy. Physical batteries convert physical energy into electrical energy.
2.What are the differences between primary batteries and secondary batteries?
The main difference lies in the active material. Secondary batteries have reversible active materials, while primary batteries do not. Secondary batteries have lower self-discharge but higher internal resistance compared to primary batteries, resulting in lower load capacity. Additionally, the mass-specific capacity and volumetric capacity of primary batteries are generally greater than rechargeable batteries.
2.What is the electrochemical principle of nickel-metal hydride (NiMH) batteries?
Nickel-metal hydride batteries use Ni-based oxides as the positive electrode, hydrogen storage alloys as the negative electrode, and an alkaline electrolyte (mainly KOH). During charging: Positive electrode reaction: Ni(OH)2 + OH- → NiOOH + H2O + e-; Negative electrode reaction: M + H2O + e- → MH+ + OH- ; During discharging: Positive electrode reaction: NiOOH + H2O + e- → Ni(OH)2 + OH-; Negative electrode reaction: MH+ + OH- → M + H2O + e-
4.What is the electrochemical principle of lithium-ion batteries?
Lithium-ion batteries use LiCoO2 as the positive electrode and carbon (C) as the negative electrode. During charging, Positive electrode reaction: LiCoO2 → Li1-xCoO2 + xLi+ + xe-; Negative electrode reaction: C + xLi+ + xe- → CLix ; Overall battery reaction: LiCoO2 + C → Li1-xCoO2 + CLix; The reverse reactions occur during discharging.
5. What are the common standards for batteries?
The commonly used standards for batteries are as follows: IEC Standards: Nickel-metal hydride (NiMH) batteries follow the IEC 61951-2:2003 standard, while the lithium-ion battery industry generally adheres to UL standards or national standards. National Standards: For NiMH batteries, the standards are GB/T 15100_1994 and GB/T 18288_2000; for lithium batteries, the standards are GB/T 10077_1998, YD/T 998_1999, and GB/T 18287_2000. Additionally, there are also Japanese Industrial Standards (JIS) related to batteries, such as JIS C standards. The IEC stands for the International Electrotechnical Commission, which is a worldwide organization composed of national electrotechnical committees. Its purpose is to promote standardization in the field of electrical and electronic technologies. IEC standards are developed by the International Electrotechnical Commission.
6. What are the main components of nickel-metal hydride batteries?
The main components of NiMH batteries include positive electrode plates (nickel oxides), negative electrode plates (hydrogen storage alloys), electrolyte (usually KOH), separator paper, sealing rings, positive electrode caps, and battery shells.
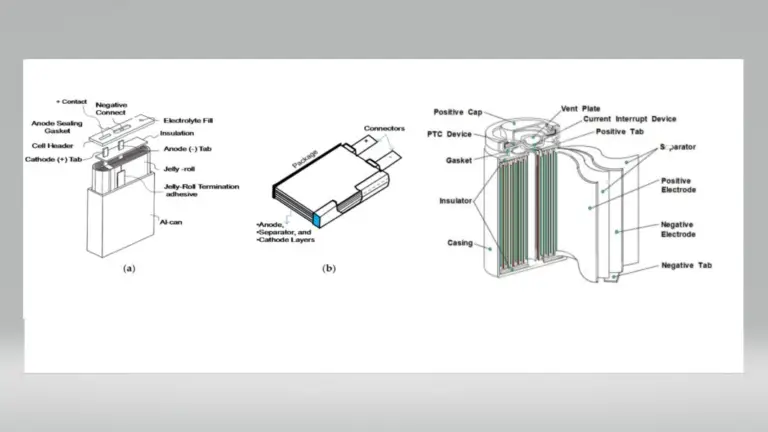
7. What are the main components of lithium-ion batteries?
Lithium-ion batteries consist of upper and lower covers, positive electrode plates (LiCoO2 as active material), separators (a special composite film), negative electrodes (carbon as active material), organic electrolytes, and battery shells (steel or aluminum).
8. What is internal resistance in batteries?
Internal resistance refers to the resistance encountered by the current flowing through the battery during operation. It consists of ohmic resistance and polarization resistance. High internal resistance leads to a decrease in discharge voltage and shortened discharge time. Internal resistance is influenced by battery materials, manufacturing processes, and battery structure, and it is an important parameter for evaluating battery performance. Note: Charging-state internal resistance is usually used as a standard. Special internal resistance meters are used to measure battery internal resistance, rather than multimeters on the ohm scale.
9. What is nominal voltage?
Nominal voltage refers to the voltage exhibited during normal operation. For example, the nominal voltage for rechargeable nickel-cadmium or nickel-metal hydride batteries is 1.2V, while for rechargeable lithium batteries, it is around 3.6V.
10. What is open circuit voltage?
Open circuit voltage is the potential difference between the positive and negative terminals of a battery when no current flows through the circuit, i.e., when the battery is not in use. Working voltage, also known as terminal voltage, refers to the potential difference between the positive and negative terminals of a battery when current flows through the circuit during operation.
11.What is battery capacity?
Battery capacity can be divided into rated capacity and actual capacity. The rated capacity of a battery refers to the minimum amount of charge that the battery should deliver under certain discharge conditions as specified or guaranteed during its design and manufacture. According to IEC standards, the rated capacity of nickel-cadmium and nickel-metal hydride batteries is determined by discharging them at 0.2C after charging at 0.1C for 16 hours, until reaching 1.0V, and is expressed in C5. For lithium-ion batteries, the rated capacity is determined by charging them at constant current (1C) to constant voltage (4.2V) for 3 hours, then discharging them at 0.2C to 2.75V, and is expressed in Ah or mAh (1Ah = 1000mAh). The actual capacity of a battery refers to the actual amount of charge it delivers under specific discharge conditions, primarily influenced by the discharge rate and temperature (thus, battery capacity should ideally be specified with charging and discharging conditions). Battery capacity is measured in Ah or mAh.
12. What is battery residual capacity?
When a rechargeable battery is discharged at a high current (such as 1C or above), the “bottleneck effect” caused by the high current rate may lead the battery to reach its endpoint voltage before completely discharging its capacity. However, it can still be discharged at a lower current, such as 0.2C, until reaching 1.0V/cell (for nickel-cadmium and nickel-metal hydride batteries) or 3.0V/cell (for lithium batteries). The capacity discharged under these conditions is called residual capacity.
13. What is discharge plateau?
The discharge plateau of nickel-metal hydride rechargeable batteries usually refers to the stable voltage range of the battery during discharge under certain discharge conditions. Its value is influenced by the discharge current; the higher the current, the lower the voltage. For lithium-ion batteries, the discharge plateau is typically measured by charging the battery to 4.2V at a low current (less than 0.01C), then letting it rest for 10 minutes before discharging it at any discharge rate until reaching 3.6V. It is an important criterion for evaluating battery performance.
Battery Marking
14.What is the identification method for rechargeable batteries specified by IEC
The identification method for rechargeable batteries specified by IEC consists of five parts for nickel-metal hydride (NiMH) batteries:
- Battery type: HF, HR for nickel-metal hydride batteries.
- Battery size data: Includes diameter for round batteries, height for round and square batteries, width, and thickness for square batteries. These values are separated by slashes and are in millimeters.
- Discharge characteristic symbol: L for discharge current rate within 0.5C, M for 0.5-3.5C, H for 3.5-7.0C, and X for high-rate discharge from 7C-15C.
- High-temperature battery symbol: Indicated by the letter T.
- Battery connection tab symbol: CF for no tab, HH for flat tab for series connection, HB for staggered tab for parallel connection.
For example, HF18/07/49 represents a square NiMH battery with a width of 18mm, thickness of 7mm, and height of 49mm. KRMT33/62HH represents a nickel-cadmium battery with a discharge rate between 0.5C-3.5C, high-temperature series single cell (flat tab), a diameter of 33mm, and a height of 62mm.
According to IEC 61960 standard, the identification for secondary lithium-ion batteries is as follows:
- Battery identification composition: 3 letters followed by 5 digits (cylindrical) or 6 digits (prismatic).
- First letter: Indicates the negative electrode material. I – indicates lithium-ion with built-in battery; L – indicates lithium metal or lithium alloy electrode.
- Second letter: Indicates the positive electrode material. C – cobalt-based electrode; N – nickel-based electrode; M – manganese-based electrode; V – vanadium-based electrode.
- Third letter: Indicates the battery shape. R – cylindrical battery; L – prismatic battery.
- Numbers: For cylindrical batteries, 5 digits represent the diameter and height. The units are in millimeters for diameter and tenths of millimeters for height. If either dimension is 100mm or more, a slash should be added between the dimensions. For prismatic batteries, 6 digits represent the thickness, width, and height in millimeters. If any dimension is 100mm or more, a slash should be added between dimensions. If any dimension is less than 1mm, the letter “t” should be added before that dimension, and the unit is tenths of millimeters.
For example, ICR18650 represents a cylindrical secondary lithium-ion battery with cobalt positive electrode material, approximately 18mm in diameter and 65mm in height. ICR20/1050. ICP083448 represents a prismatic secondary lithium-ion battery with cobalt positive electrode material, approximately 8mm in thickness, 34mm in width, and 48mm in height. ICP08/34/150 represents a prismatic secondary lithium-ion battery with cobalt positive electrode material, approximately 8mm in thickness, 34mm in width, and 150mm in height. ICPt73448 represents a prismatic secondary lithium-ion battery with cobalt positive electrode material, approximately 0.7mm in thickness, 34mm in width, and 48mm in height.
15. What are the packaging materials for batteries?
Non-drying intermediaries (paper) such as fiber paper, double-sided adhesive
PVC film, trademark tubes
Connection tabs: stainless steel sheets, pure nickel sheets, nickel-plated steel sheets
Lead-out tabs: stainless steel sheets (easy to solder) Pure nickel sheets (strong soldering points)
Plug types
Protective components such as temperature control switches, overcurrent protectors, current limiting resistors
Cardboard boxes, paper boxes
Plastic shell types
16. What is the purpose of battery packaging, assembly, and design?
Aesthetic appearance, branding
Voltage limitation of batteries; to achieve higher voltage, multiple batteries need to be connected in series
Battery protection to prevent short circuits and extend battery lifespan
Size limitations
Ease of transportation
Design for special functions, such as waterproofing, special exterior designs, etc.
Battery performances and tests
17. What are the main aspects of performance for secondary batteries?
They mainly include voltage, internal resistance, capacity, energy density, internal pressure, self-discharge rate, cycle life, sealing performance, safety performance, storage performance, appearance, etc. Other aspects include overcharging, overdischarging, corrosion resistance, etc.
18. What are the reliability test items for batteries?
Cycle life
Discharge characteristics at different rates
Discharge characteristics at different temperatures
Charging characteristics
Self-discharge characteristics
Storage characteristics
Overdischarge characteristics
Internal resistance characteristics at different temperatures
Temperature cycling test
Drop test
Vibration test
Capacity test
Internal resistance test
GMS test
High and low temperature impact test
Mechanical impact test
High temperature and humidity test
19. What are the safety test items for batteries?
- Short circuit test
- Overcharge and overdischarge test
- Dielectric strength test
- Impact test
- Vibration test
- Heating test
- Fire test
- Temperature cycling test
- Trickle charging test
- Free fall test
- Low pressure test
- Forced discharge test
- Electric heating plate test
- Thermal shock test
- Needle puncture test
- Compression test
- Heavy object impact test
20.What are the common charging methods?
Charging methods for nickel-metal hydride batteries:
Constant current charging: the charging current remains constant throughout the charging process, which is the most common method.
Constant voltage charging: the voltage across the battery remains constant during charging, and the current in the circuit gradually decreases as the battery voltage increases.
Constant current constant voltage charging: the battery is first charged with a constant current (CC), and when the battery voltage reaches a certain value, the voltage remains constant (CV), and the current in the circuit decreases to a very small level, ultimately approaching 0.
Charging method for lithium-ion batteries: Constant current constant voltage charging: the battery is first charged with a constant current (CC), and when the battery voltage reaches a certain value, the voltage remains constant (CV), and the current in the circuit decreases to a very small level, ultimately approaching 0.
21.What is the standard charge and discharge for nickel-metal hydride batteries?
The IEC international standard specifies the standard charge and discharge for nickel-metal hydride batteries as follows: first, discharge the battery at 0.2C to 1.0V per cell, then charge it at 0.1C for 16 hours, let it stand for 1 hour, and then discharge it at 0.2C to 1.0V per cell, which is considered the standard charge and discharge for the battery.
22.What is pulse charging? How does it affect battery performance?
Pulse charging generally uses a charging and discharging method, where the battery is charged for 5 seconds and then discharged for 1 second. This charging process reduces most of the oxygen generated during charging under discharge pulses back to the electrolyte. This not only limits the gasification of the internal electrolyte but also helps restore or approximate the original capacity of heavily polarized old batteries after using this charging method for 5-10 charge-discharge cycles.
23. What is trickle charging?
Trickle charging is used to compensate for capacity losses due to self-discharge after the battery is fully charged. Generally, pulse current charging is used to achieve this purpose.
24.What is charging efficiency?
Charging efficiency refers to the degree to which the electrical energy consumed during charging is converted into chemical energy stored in the battery during the charging process. It is mainly affected by battery technology and the working environment temperature. Generally, the higher the ambient temperature, the lower the charging efficiency.
25. What is discharging efficiency?
Discharging efficiency refers to the ratio of the actual amount of electricity discharged to the rated capacity when the battery is discharged to the endpoint voltage under certain discharge conditions. It is mainly affected by discharge rate, ambient temperature, internal resistance, and other factors. Generally, the higher the discharge rate, the lower the discharging efficiency. Lower temperatures also lead to lower discharging efficiency.
26. What is battery output power?
Battery output power refers to the ability to deliver energy in a unit of time. It is calculated based on the discharge current (I) and discharge voltage (U), P=U*I, measured in watts. The smaller the internal resistance of the battery, the higher the output power. The battery’s internal resistance should be smaller than that of the appliance it powers; otherwise, the battery’s power consumption will be greater than that of the appliance, which is uneconomical and may damage the battery.
27. What is self-discharge of secondary batteries? What are the self-discharge rates for different types of batteries?
Self-discharge, also known as charge retention capacity, refers to the ability of a battery to retain its charge under certain environmental conditions in an open-circuit state. Generally, self-discharge is influenced by manufacturing processes, materials, and storage conditions. Self-discharge is one of the main parameters for measuring battery performance. Generally, the lower the storage temperature of the battery, the lower the self-discharge rate. However, it should also be noted that both excessively low and high temperatures can potentially damage the battery, rendering it unusable.
A certain degree of self-discharge after the battery is fully charged and left open-circuit for a period of time is considered normal. According to IEC standards, nickel-metal hydride batteries are left open-circuit for 28 days at a temperature of 20°C ± 5°C and a humidity of (65 ± 20)%, and their discharge capacity reaches 60% of the initial capacity at 0.2C discharge.
28. What is the 24-hour self-discharge test?
For lithium batteries:
The 24-hour self-discharge test is commonly used to quickly assess their charge retention capacity. The battery is discharged at 0.2C to 3.0V, then charged at a constant current and constant voltage of 1C to 4.2V, with a cutoff current of 10mA. After resting for 15 minutes, it is discharged at 1C to 3.0V to measure its discharge capacity C1. The battery is then charged again at a constant current and constant voltage of 1C to 4.2V, with a cutoff current of 10mA, and after resting for 24 hours, the 1C capacity C2 is measured. The ratio of C2 to C1 multiplied by 100% should be greater than 99%.
29. What is the difference between internal resistance in the charged state and the discharged state?
Internal resistance in the charged state refers to the resistance of the battery when it is fully charged at 100%. Internal resistance in the discharged state refers to the resistance of the battery after being fully discharged.
Generally, the resistance in the discharged state is less stable and tends to be higher, while the resistance in the charged state is lower and more stable. In the course of battery usage, only the resistance in the charged state is practically meaningful. In the later stages of battery usage, due to the depletion of the electrolyte and the reduction in the activity of internal chemical substances, the internal resistance of the battery may increase to varying degrees.
30. What is static resistance? What is dynamic resistance?
Static resistance refers to the resistance of the battery during discharge, while dynamic resistance refers to the resistance of the battery during charging.
31. What is the standard overcharge resistance test?
IEC specifies the standard overcharge resistance test for nickel-metal hydride batteries as follows:
The battery is discharged at 0.2C to 1.0V/cell, then continuously charged at 0.1C for 48 hours. The battery should show no deformation or leakage, and after overcharging, the time for 0.2C discharge to 1.0V should be greater than 5 hours.
32. What is the IEC standard cycle life test?
IEC specifies the standard cycle life test for nickel-metal hydride batteries as follows:
After discharging the battery at 0.2C to 1.0V/cell: 01) Charge at 0.1C for 16 hours, then discharge at 0.2C for 2 hours and 30 minutes (one cycle).
- Charge at 0.25C for 3 hours and 10 minutes, then discharge at 0.25C for 2 hours and 20 minutes (cycles 2-48).
- Charge at 0.25C for 3 hours and 10 minutes, then discharge to 1.0V at 0.25C (cycle 49).
- Charge at 0.1C for 16 hours, rest for 1 hour, then discharge at 0.2C to 1.0V (cycle 50). For nickel-metal hydride batteries, repeat cycles 1-4 for a total of 400 cycles, and the time for 0.2C discharge should be greater than 3 hours. For nickel-cadmium batteries, repeat cycles 1-4 for a total of 500 cycles, and the time for 0.2C discharge should be greater than 3 hours.
33. What is internal pressure in batteries?
Internal pressure in batteries refers to the internal gas pressure of the battery, which is caused by gases generated during charging and discharging in sealed batteries. It is mainly influenced by battery materials, manufacturing processes, battery structure, etc. The main cause is the accumulation of gases generated by the decomposition of water and organic solvents inside the battery. Generally, battery internal pressure is maintained at a normal level. However, in cases of overcharging or overdischarging, the internal pressure of the battery may increase. For example, during overcharging, the reaction at the positive electrode is: 4OH- – 4e → 2H2O + O2↑. The generated oxygen gas reacts with hydrogen gas released at the negative electrode to form water: 2H2 + O2 → 2H2O. If the rate of reaction 2 is lower than that of reaction 1, the generated oxygen gas may accumulate, leading to an increase in internal pressure.
34. What is the standard charge retention test?
IEC specifies the standard charge retention test for nickel-metal hydride batteries as follows:
After discharging the battery at 0.2C to 1.0V, charge at 0.1C for 16 hours. Then, under temperature conditions of 20°C ± 5°C and humidity of 65% ± 20%, store the battery for 28 days. Afterward, discharge at 0.2C to 1.0V again, and the nickel-metal hydride battery should last more than 3 hours.
For lithium batteries, according to national standards (as there is no relevant IEC standard), discharge the battery at 0.2C to 3.0V/cell, then charge at a constant current and constant voltage of 1C to 4.2V, with a cutoff current of 10mA. Store the battery for 28 days under temperature conditions of 20°C ± 5°C, then discharge at 0.2C to 2.75V and compare the discharge capacity with the nominal capacity. It should not be less than 85% of the initial capacity.
35. What is a short circuit test?
A short circuit test involves connecting the positive and negative terminals of a fully charged battery with a wire with an internal resistance of ≤100mΩ in an explosion-proof box. The battery should not explode or catch fire.
36. What is the high-temperature and high-humidity test?
For nickel-metal hydride batteries, the high-temperature and high-humidity test involves storing the fully charged battery under constant temperature and humidity conditions for a certain number of days and observing for any leakage during the storage period.
For lithium batteries (according to national standards), the high-temperature and high-humidity test is conducted as follows:
- Charge the battery at a constant current and constant voltage of 1C to 4.2V, with a cutoff current of 10mA.
- Place the battery in a constant temperature and humidity chamber with a temperature of (40 ± 2)°C and a relative humidity of 90% – 95% for 48 hours.
- Remove the battery and let it rest for 2 hours at (20 ± 5)°C, observing for any abnormalities in appearance.
- Discharge the battery at 1C constant current to 2.75V, then under (20 ± 5)°C conditions, conduct 1C charge and discharge cycles until the discharge capacity is not less than 85% of the initial capacity, with no more than 3 cycles.
37. What is the temperature rise test?
The temperature rise test involves placing the fully charged battery in an oven and increasing the temperature at a rate of 5°C/min from room temperature until the oven temperature reaches 130°C, maintaining it for 30 minutes. The battery should not explode or catch fire during this test.
38. What is the temperature cycling test?
The temperature cycling test consists of 27 cycles, each comprising the following steps: 01) Place the battery at room temperature in conditions of 66±3°C and 15±5% humidity for 1 hour.
- Change to conditions of 33±3°C and 90±5% humidity for 1 hour.
- Change to conditions of -40±3°C for 1 hour.
- Let the battery rest at 25°C for 0.5 hours.
After completing these 4 steps, which constitute one cycle, the battery should show no leakage, alkali climbing, rusting, or other abnormalities after undergoing all 27 cycles.
39. What is the drop test?
The drop test involves dropping the fully charged battery or battery pack three times from a height of 1 meter onto concrete (or cement) ground to simulate random impact directions.
40. What is the vibration test?
For nickel-metal hydride batteries, the vibration test method involves discharging the battery at 0.2C to 1.0V, charging at 0.1C for 16 hours, and resting for 24 hours before subjecting it to the following conditions:
- Amplitude: 0.8mm
- Vibrate the battery between 10Hz and 55Hz, increasing or decreasing the vibration rate by 1Hz per minute.
- Voltage variation of the battery should be within ±0.02V, and the internal resistance variation should be within ±5mΩ during the vibration time of 90 minutes.
For lithium batteries, the vibration test method involves discharging the battery at 0.2C to 3.0V, charging at 1C constant current and constant voltage to 4.2V, with a cutoff current of 10mA. After resting for 24 hours, subject the battery to the following conditions:
- Vibrate the battery in a cycle of vibration frequency from 10Hz to 60Hz and back to 10Hz within 5 minutes, with an amplitude of 0.06 inches. Vibrate the battery in three-axis directions for half an hour each axis.
- Voltage variation of the battery should be within ±0.02V, and the internal resistance variation should be within ±5mΩ.
41. What is the impact test?
After the battery is fully charged, place a hard rod horizontally on the battery and drop a 20-pound weight from a certain height to hit the hard rod. The battery should not explode or catch fire.
42. What is the penetration test?
After the battery is fully charged, use a nail with a certain diameter to penetrate the center of the battery and leave the nail inside the battery. The battery should not explode or catch fire.
43. What is the burning test?
Place the fully charged battery on a heating device with a special protective cover for burning, and no fragments should penetrate the protective cover.
Battery Common Issues and Analysis
44. What certifications has the company's product obtained?
The company’s products have obtained ISO9001:2000 Quality Management System certification and ISO14001:2004 Environmental Management System certification. The products have also received EU CE certification and North American UL certification, passed SGS environmental testing, and obtained patent licensing from Ovonic. Additionally, the company’s products are insured globally by PICC.
45. What is a Ready-To-Use battery?
This type of product has the following significant features compared to similar products:
- Lower self-discharge rate;
- Longer storage time;
- Tolerance to overcharging;
- Long cycle life;
- Especially effective in recovering capacity when the battery voltage drops below 1.0V; More importantly, this type of battery can maintain a charge retention rate of up to 75% after one year of storage at 25°C. Therefore, it is considered the ideal product to replace disposable batteries.
46. Why is Ready-To-Use (HFR) considered the ideal product to replace disposable batteries?
- This type of product has the following significant features compared to similar products:
- Lower self-discharge rate;
- Longer storage time;
- Tolerance to overcharging;
- Long cycle life;
- Especially effective in recovering capacity when the battery voltage drops below 1.0V; More importantly, this type of battery can maintain a charge retention rate of up to 75% after one year of storage at 25°C. Therefore, it is considered the ideal product to replace disposable batteries.
47. What are the precautions for battery usage?
- Carefully read the battery manual before use;
- Ensure that the contacts between the appliance and the battery are clean, wipe them with a damp cloth if necessary, and insert them according to the polarity markings after drying;
- Do not mix old and new batteries, or different types of batteries of the same model to avoid reducing efficiency;
- Do not attempt to regenerate disposable batteries through heating or charging methods;
- Avoid short-circuiting the battery;
- Do not disassemble, heat, or immerse the battery in water;
- Remove the batteries from appliances that are not in use for a long time, and turn off the switch after use;
- Dispose of waste batteries properly, separate them from other garbage as much as possible to avoid environmental pollution;
- Keep batteries out of reach of children and do not let them replace batteries without adult supervision;
- Store batteries in a cool, dry place away from direct sunlight.
48. What are the differences between common rechargeable batteries?
- Currently, nickel-cadmium (Ni-Cd), nickel-metal hydride (Ni-MH), and lithium-ion rechargeable batteries are widely used in various portable electronic devices (such as laptops, cameras, and mobile phones). Each type of rechargeable battery has its unique chemical properties. The main differences between nickel-cadmium and nickel-metal hydride batteries are: nickel-metal hydride batteries have higher energy density. Compared to the same model of nickel-cadmium batteries, nickel-metal hydride batteries have twice the capacity. This means that using nickel-metal hydride batteries can significantly extend the working time of devices without adding extra weight. Another advantage of nickel-metal hydride batteries is that they greatly reduce the “memory effect” present in nickel-cadmium batteries, making nickel-metal hydride batteries more convenient to use. Nickel-metal hydride batteries are also more environmentally friendly than nickel-cadmium batteries because they do not contain toxic heavy metal elements. Li-ion batteries have also become the standard power source for portable devices. Li-ion batteries can provide the same energy as nickel-metal hydride batteries but can reduce weight by about 35%. This is crucial for devices such as cameras and laptops. The lack of “memory effect” and absence of toxic substances are also important factors that make Li-ion batteries the standard power source.
The discharge efficiency of nickel-metal hydride batteries significantly decreases at low temperatures, and the charging efficiency generally increases with temperature. However, when the temperature exceeds 45°C, the performance of charging battery materials will degrade, leading to a significant reduction in battery cycle life.
49. What is the rate discharge of a battery? What is the hour rate discharge of a battery?
Rate discharge refers to the relationship between the discharge current (A) and the rated capacity (A•h) during discharge. Hour rate discharge refers to the number of hours required to discharge the rated capacity at a certain output current.
50. Why is it necessary to keep the battery warm when shooting in winter?
In cold weather, the activity of active substances in digital camera batteries significantly decreases, which may lead to the inability to provide normal operating current for the camera. Therefore, when shooting outdoors in low-temperature areas, it is important to ensure the warmth of the camera or battery.
51. What is the operating temperature range of lithium-ion rechargeable batteries?
Charging: -10 to 45°C Discharging: -30 to 55°C
52. Can batteries of different capacities be combined together?
Combining batteries of different capacities or mixing new and old batteries may lead to leakage, zero voltage, and other issues. This is because during the charging process, differences in capacity can result in some batteries being overcharged while others are not fully charged. During discharge, batteries with higher capacity may not discharge completely, while those with lower capacity may be over-discharged. This vicious cycle can damage the batteries, leading to leakage or low (zero) voltage.
53. What is an external short circuit, and how does it affect battery performance?
An external short circuit occurs when both ends of a battery are connected to any conductor. The impact of an external short circuit on battery performance can vary depending on the battery type. It can lead to consequences such as increased electrolyte temperature and internal pressure. If the pressure exceeds the pressure resistance value of the battery cap, the battery may leak, causing severe damage. In cases where the safety valve fails, it may even lead to an explosion. Therefore, never short-circuit batteries externally.
54. What are the main factors affecting battery lifespan?
- Charging: When selecting a charger, it’s best to use one with proper charging termination features (such as overcharge protection, negative voltage cutoff (-dV), and overheating protection) to avoid shortening the battery’s lifespan due to overcharging. Generally, slow charging extends battery lifespan more than fast charging.
- Discharging: a. The depth of discharge is a major factor affecting battery lifespan. The deeper the discharge, the shorter the battery lifespan. In other words, reducing the depth of discharge significantly extends the battery’s lifespan. Therefore, avoid discharging the battery to extremely low voltages. b. Discharging the battery at high temperatures can shorten its lifespan. c. If electronic equipment cannot completely stop all current flow, leaving the equipment unused for a long time without removing the battery can lead to excessive battery drain, resulting in over-discharge. d. Mixing batteries with different capacities, chemical structures, or different charge levels, as well as mixing new and old batteries, can lead to excessive discharge and even reverse charging.
- Storage: Storing batteries at high temperatures for extended periods can cause electrode activity decay, shortening the battery’s lifespan.
55. Can batteries be stored in electrical appliances after use or for long periods of non-use?
It’s better to remove the batteries from electrical appliances if they won’t be used for an extended period. Store the batteries in a cool, dry place. Even if the appliance is turned off, the system may still draw a low current from the batteries, which can shorten their lifespan.
56. What are the optimal conditions for storing batteries? Is it necessary to fully charge batteries for long-term storage?
According to IEC standards, batteries should be stored at a temperature of 20°C ± 5°C and a humidity of (65±20)%. Generally, the higher the storage temperature of the battery, the lower the remaining capacity rate, and vice versa. The best place to store batteries, especially primary batteries, is in a refrigerator at temperatures between 0°C to 10°C. Secondary batteries, even if they lose capacity after storage, can be restored by recharging and discharging a few times.
In theory, batteries will always experience energy loss during storage. The inherent electrochemical structure of batteries results in inevitable capacity loss, mainly due to self-discharge. Self-discharge is typically related to the solubility of the positive electrode material in the electrolyte and its instability (self-decomposition) when heated. Rechargeable batteries have much higher self-discharge rates compared to primary batteries.
For long-term battery storage, it’s best to keep them in a dry and cool environment with the remaining battery capacity at around 40%. Additionally, it’s recommended to use the batteries at least once a month to maintain their good condition and prevent complete discharge that may damage the batteries.
57. What is a standard battery?
A standard battery is internationally recognized as the potential (voltage) measurement standard. It was invented by the American electrical engineer E. Weston in 1892, hence it’s also called the Weston cell.
The positive electrode of a standard battery is a mercury (II) sulfate electrode, the negative electrode is a cadmium-mercury amalgam (containing 10% or 12.5% cadmium), and the electrolyte is an acidic saturated cadmium sulfate solution, which is essentially a saturated solution of cadmium sulfate and mercury (II) sulfate.
58. What are the possible reasons for a single battery to show zero voltage or low voltage?
- External short circuit or overcharging, reverse charging (forced over-discharge).
- Continuous overcharging with high-rate large currents, causing the battery core to expand and direct contact between positive and negative electrodes leading to a short circuit.
- Internal short circuit or micro-short circuit, such as improper placement of positive and negative electrode sheets causing them to contact and short-circuit or improper contact of positive electrode sheets.
59. What are the possible reasons for a battery pack to show zero voltage or low voltage?
- Whether a single cell has zero voltage.
- Short circuit or open circuit in the plug, poor connection with the plug.
- Leads detached or poorly soldered from the battery.
- Incorrect internal battery connection, soldering, or detachment between connection sheets and batteries.
- Incorrect connection of internal electronic components in the battery pack, causing damage.
60. What are the methods to prevent overcharging of batteries?
To prevent overcharging of batteries, it’s necessary to control the charging endpoint. When the battery is fully charged, special information can be utilized to determine if the charging has reached the endpoint. Generally, there are six methods to prevent battery overcharging:
- Peak voltage control: Determine the charging endpoint by detecting the peak voltage of the battery.
- ΔT/Δt control: Determine the charging endpoint by detecting the rate of change of peak temperature of the battery.
- ΔT control: When the battery is fully charged, the difference between its temperature and ambient temperature will reach its maximum.
- -ΔV control: When the battery reaches a peak voltage after being fully charged, the voltage will drop by a certain amount.
- Timer control: Control the charging endpoint by setting a certain charging time, usually set to charge for the time required to charge to 130% of the rated capacity.
61. What are the possible reasons why batteries or battery packs cannot be charged?
- Zero voltage in the battery or zero voltage battery in the battery pack.
- Incorrect connection of the battery pack, internal electronic components, or abnormal protection circuit.
- Malfunction of the charging equipment with no output current.
- External factors leading to too low charging efficiency (such as extremely low or high temperatures).
62. What are the possible reasons why batteries or battery packs cannot discharge?
- Lifespan deterioration of the battery after storage or use.
- Insufficient or no charging.
- Very low ambient temperature.
- Low discharge efficiency, such as during high current discharge where the diffusion rate of internal materials in ordinary batteries cannot keep up with the reaction rate, causing a rapid voltage drop and inability to discharge.
63. What are the possible reasons for short discharge time of batteries or battery packs?
- Battery not fully charged, insufficient charging time, low charging efficiency, etc.
- Excessive discharge current leading to decreased discharge efficiency and shortened discharge time.
- Very low ambient temperature during discharge causing decreased discharge efficiency.
64. What is overcharging, and what impact does it have on battery performance?
Overcharging refers to continuing to charge a battery after it has been fully charged through a certain charging process. For Ni-MH batteries, overcharging leads to the following reactions: Positive electrode: 4OH- – 4e → 2H2O + O2↑; (1) Negative electrode: 2H2 + O2 → 2H2O (2)
Since the negative electrode capacity is generally higher than the positive electrode capacity in design, the oxygen generated at the positive electrode combines with the hydrogen generated at the negative electrode through the separator paper, so the internal pressure of the battery will not significantly increase under normal circumstances. However, if the charging current is too high or the charging time is too long, the generated oxygen may not be consumed in time, leading to increased internal pressure, battery deformation, leakage, and other adverse phenomena. Additionally, its electrical performance will significantly decrease.
65. What is overdischarging, and what impact does it have on battery performance?
Overdischarging occurs when a battery continues to discharge after depleting the stored energy to a certain level. The cutoff voltage for discharging is usually determined based on the discharge current, with 0.2C-2C discharge generally set at 1.0V per cell, and for discharges above 3C such as 5C or 10C, it is set at 0.8V per cell. Overdischarging can have catastrophic consequences for the battery, especially with high current overdischarge or repeated overdischarge, resulting in significant damage to the reversibility of active materials in the positive and negative electrodes. Even with recharging, only partial recovery is possible, and the capacity will also significantly deteriorate.
66. What are the main reasons for the swelling of rechargeable batteries?
- Poor battery protection circuit.
- Battery without protection function leading to cell swelling.
- Poor performance of the charger, excessive charging current causing battery swelling.
- Battery subjected to continuous overcharging with high current rates.
- Battery subjected to forced overdischarge.
- Design issues with the battery itself.
67. What is battery explosion, and how can battery explosions be prevented?
Battery explosion refers to the instantaneous discharge of solid substances from any part inside the battery, propelled to a distance of more than 25cm away from the battery. General preventive measures include: 01) Avoid overcharging and short circuits.
- Use high-quality charging equipment for charging.
- Ensure that the battery’s ventilation holes remain unobstructed.
- Pay attention to heat dissipation when using the battery.
- Prohibit the mixing of batteries of different types, ages, or conditions.
68. Types and Pros/Cons of Battery Protection Components:
Here is a comparison of several common battery protection components:

69. What is a portable battery?
Portable means easy to carry and convenient to use. Portable batteries are mainly used to provide power for handheld and cordless devices. Larger batteries (e.g., 4 kilograms or more) do not belong to portable batteries. Nowadays, typical portable batteries weigh a few hundred grams.
The family of portable batteries includes primary batteries and rechargeable batteries (secondary batteries). Button cells belong to a special group among them.
70. What are the characteristics of rechargeable portable batteries?
Every battery is an energy converter that can directly convert stored chemical energy into electrical energy. For rechargeable batteries, this process can be described as follows: during charging, electrical energy is converted into chemical energy, which is then converted back into electrical energy during discharge. Secondary batteries can undergo this cycle over 1000 times.
Rechargeable portable batteries exist in different electrochemical types, including lead-acid (2V per cell), nickel-cadmium (1.2V per cell), nickel-metal hydride (1.2V per cell), and lithium-ion batteries (3.6V per cell). The typical characteristic of these batteries is a relatively constant discharge voltage (a voltage plateau during discharge), with a rapid voltage drop at the beginning and end of discharge.
71. Can any charger be used for rechargeable portable batteries?
No, because each charger corresponds to a specific charging process and electrochemical reaction, such as lithium-ion, lead-acid, or Ni-MH batteries. They not only have different voltage characteristics but also different charging modes. Only specially developed fast chargers can provide the most suitable charging effect for Ni-MH batteries. Slow chargers can be used when urgently needed, but they require more time. It should be noted that although some chargers have qualified labels, they should be used with caution as chargers for different electrochemical systems may not yield satisfactory results and can be dangerous.
72. Can rechargeable 1.2V portable batteries replace 1.5V alkaline-manganese batteries?
Alkaline-manganese batteries have a discharge voltage range of 1.5V to 0.9V, while rechargeable batteries have a constant discharge voltage of 1.2V per cell. This voltage is roughly equivalent to the average voltage of alkaline-manganese batteries, so it is feasible to use rechargeable batteries instead. The reverse is also true.
73. What are the advantages and disadvantages of rechargeable batteries?
The advantages of rechargeable batteries include long service life, which makes them economically viable despite being more expensive than primary batteries in the long run. Rechargeable batteries also have a higher load capacity than most primary batteries. However, ordinary secondary batteries have a relatively high self-discharge rate, which can cause inconvenience during use as it is difficult to predict when the discharge will end. Lithium-ion batteries, on the other hand, can provide longer usage time for camera equipment, have high load capacity, high energy density, and the voltage drop during discharge weakens as the discharge progresses.
The disadvantages of rechargeable batteries include the difficulty in predicting the end of discharge due to the nearly constant discharge voltage of ordinary secondary batteries, making them unsuitable for certain applications. Lithium-ion batteries are almost ideal due to their low self-discharge rate, but they require strict charging and discharging conditions to ensure longevity.
74. What are the advantages of nickel-metal hydride (Ni-MH) batteries? What are the advantages of lithium-ion batteries?
The advantages of Ni-MH batteries are: 01) Low cost;
- Good fast-charging performance;
- Long cycle life;
- No memory effect;
- No pollution, environmentally friendly batteries;
- Wide temperature range;
- Good safety performance.
The advantages of lithium-ion batteries are: 01) High energy density;
- High operating voltage;
- No memory effect;
- Long cycle life;
- Pollution-free;
- Lightweight;
- Low self-discharge rate.
75. What are the advantages of lithium iron phosphate (LiFePO4) batteries?
LiFePO4 batteries are mainly used in power batteries, and their advantages are mainly reflected in the following aspects: 01) Ultra-long service life;
- Safe to use;
- Fast charging and discharging with high currents;
- High temperature resistance;
- Large capacity;
- No memory effect;
- Small size and lightweight;
- Environmentally friendly.
76. What are the advantages of lithium polymer batteries?
- No leakage issues: Lithium polymer batteries do not contain liquid electrolytes internally; instead, they use a gel-like solid.
- Thin design: With a capacity of 3.6V and 400mAh, the thickness of lithium polymer batteries can be as thin as 0.5mm.
- Flexible design: Lithium polymer batteries can be designed in various shapes.
- Bendable and deformable: Polymer batteries can bend up to around 900 degrees.
- High single-cell voltage: Unlike liquid electrolyte batteries that require multiple cells in series for high voltage, polymer batteries can achieve high voltage within a single cell.
- Multilayer configuration: Due to the absence of liquid, multiple layers can be combined within a single cell to achieve high voltage.
- Higher capacity: Lithium polymer batteries can offer twice the capacity of lithium-ion batteries of the same size.
77. What is the principle of a charger? What are the main types?
A charger is a static rectifier device that uses power electronic semiconductor devices to convert fixed-voltage and frequency alternating current (AC) into direct current (DC). There are many types of chargers, including lead-acid battery chargers, valve-regulated lead-acid battery testing and monitoring chargers, nickel-cadmium battery chargers, nickel-metal hydride battery chargers, lithium-ion battery chargers, portable electronic device lithium-ion battery chargers, lithium-ion battery protection circuit multi-function chargers, electric vehicle battery chargers, and more.
Battery Types and Applications
78. How are batteries classified?
Chemical Batteries:
- Primary Batteries: Dry batteries (carbon-zinc dry batteries), alkaline-manganese batteries, lithium batteries, activated batteries, zinc-mercury batteries, cadmium-mercury batteries, zinc-air batteries, zinc-silver batteries, solid-state electrolyte batteries (silver-iodine batteries), etc.
- Secondary Batteries: Lead-acid batteries, nickel-cadmium batteries (Ni-Cd batteries), nickel-metal hydride batteries (Ni-MH batteries), lithium-ion batteries (Li-ion batteries), sodium-sulfur batteries, etc.
- Other Batteries: Fuel cell batteries, air batteries, paper batteries, light batteries, nano batteries, etc.
Physical Batteries:
- Solar Cells (solar cell batteries)
79. What battery will dominate the battery market?
As multimedia devices such as cameras, mobile phones, cordless phones, laptops, etc., which incorporate images or sound, become increasingly important in household appliances, secondary batteries are extensively used in these areas compared to primary batteries. Secondary rechargeable batteries will continue to evolve towards smaller size, lighter weight, higher capacity, and intelligence.
80. What is a smart secondary battery?
A smart battery is equipped with a chip that not only provides power to the device but also controls its major functions. This type of battery can display remaining capacity, number of cycles, temperature, etc. However, smart batteries are not yet widely available in the market, but they are expected to dominate the market in the future, especially in portable cameras, cordless phones, mobile phones, and laptops.
81. What is a paper battery?
A paper battery is a new type of battery composed of electrodes, electrolytes, and a separator. Specifically, this new type of paper battery is made up of cellulose paper implanted with electrodes and electrolytes, where the cellulose paper acts as a separator. The electrodes include carbon nanotubes embedded in cellulose and metallic lithium covered by a thin film made of cellulose. The electrolyte used is a solution of lithium hexafluorophosphate. This battery can be folded and has a thickness equivalent to that of a piece of paper. Researchers believe that due to its various properties, this paper battery will become a new type of energy storage device.
82. What is a photovoltaic cell?
A photovoltaic cell is a semiconductor device that generates an electromotive force under illumination. There are many types of photovoltaic cells, including selenium photovoltaic cells, silicon photovoltaic cells, and thallium sulfide and silver sulfide photovoltaic cells. They are mainly used in instruments, automatic telemetry, and remote control systems. Some photovoltaic cells can directly convert solar energy into electrical energy, which is also known as a solar cell.
83. What is a solar cell? What are the advantages of solar cells?
A solar cell is a device that converts light energy, primarily sunlight, into electrical energy. It operates based on the principle of the photovoltaic effect, where the internal electric field of a PN junction separates photo-generated charge carriers to create a photovoltage across the junction, which generates power when connected to an external circuit. The power output of a solar cell is related to the intensity of sunlight; the stronger the sunlight, the higher the power output.
Solar energy systems are easy to install, expand, and dismantle. They are also cost-effective and do not consume energy during operation. Additionally, these systems are resistant to mechanical wear. Solar cells have several advantages, including: 01) High charge absorption capacity;
- Long cycle life;
- Good recharging performance;
- No maintenance required.
84. What is a fuel cell? How are fuel cells classified?
A fuel cell is an electrochemical system that directly converts chemical energy into electrical energy. The most common classification method for fuel cells is based on the type of electrolyte used. According to this, fuel cells can be classified into alkaline fuel cells (using potassium hydroxide as the electrolyte), phosphoric acid fuel cells (using concentrated phosphoric acid as the electrolyte), proton exchange membrane fuel cells (using fully or partially fluorinated sulfonic acid-type proton exchange membranes as the electrolyte), molten carbonate fuel cells (using molten lithium-potassium carbonate or lithium-sodium carbonate as the electrolyte), and solid oxide fuel cells (using solid oxide as the oxygen ion conductor, such as using yttria-stabilized zirconia membranes as the electrolyte). Sometimes, fuel cells are also classified based on operating temperature, such as low-temperature fuel cells (operating temperature below 100°C), including alkaline fuel cells and proton exchange membrane fuel cells; medium-temperature fuel cells (operating temperature between 100-300°C), including phosphoric acid fuel cells; high-temperature fuel cells (operating temperature between 600-1000°C), including molten carbonate fuel cells and solid oxide fuel cells.
85. Why do fuel cells have great development potential?
In recent decades, the United States has paid special attention to the development of fuel cells, while Japan has vigorously conducted technical development based on the introduction of American technology. The reason why fuel cells have attracted attention in some developed countries is mainly due to the following advantages: 01) High efficiency: Since fuel cells directly convert chemical energy into electrical energy without going through a thermal energy conversion, their conversion efficiency is not limited by the thermodynamic Carnot cycle. They also eliminate mechanical transmission losses and maintain high conversion efficiency regardless of the size of power generation, making fuel cells highly efficient.
1. Low noise and low pollution: Fuel cells have minimal noise due to the absence of moving mechanical parts except for small moving parts in the control system. They are also low-pollution energy sources. For example, phosphoric acid fuel cells emit sulfur oxides and nitrogen oxides lower than the U.S. regulatory standards by two orders of magnitude.
- Strong adaptability: Fuel cells can use various hydrogen-containing fuels such as methane, methanol, ethanol, biogas, natural gas, and synthetic gas, while the oxidant is inexhaustible air. They can be assembled into different powers and types according to user needs and conveniently installed in the most convenient location for users. They can also be assembled into large power stations and connected to conventional power supply systems, which helps in regulating power loads.
- Short construction cycle and easy maintenance: Once fuel cell industrial production is established, various standard components of power generation devices can be continuously produced in factories. They are easy to transport and assemble at power generation sites. It has been estimated that the maintenance volume of a 40 kW phosphoric acid fuel cell is only 25% of that of a diesel generator with the same power. Due to these advantages, both the United States and Japan attach great importance to the development of fuel cells.
86. What is a nanobattery?
A nanobattery refers to a battery made using nanomaterials (such as nano MnO2, LiMn2O4, Ni(OH)2, etc.). Nanomaterials have unique microstructures and physical and chemical properties (such as quantum size effects, surface effects, and tunneling quantum effects). Currently, the mature nanobattery technology in China is the nanocomposite carbon fiber battery. It is mainly used in electric cars, electric motorcycles, and electric-assist bicycles. This type of battery can be charged and discharged up to 1000 cycles, with continuous use for about 10 years. It only takes about 20 minutes for a full charge, with a range of 400 km on flat roads and a weight of 128 kg, surpassing the battery car levels in countries like the United States and Japan, whose nickel-metal hydride batteries require approximately 6-8 hours to charge for a range of 300 km on flat roads.
87. What is a plastic lithium-ion battery?
The so-called plastic lithium-ion battery refers to a lithium-ion battery that uses ion-conducting polymers as electrolytes. These polymers can be in solid-state or gel-state.
88. Where are rechargeable batteries best used?
Rechargeable batteries are particularly suitable for electrical devices that require relatively high energy supply or demand large current discharge, such as portable radios, CD players, small radios, electronic game consoles, electric toys, household appliances, professional cameras, mobile phones, cordless phones, laptops, and other devices that require higher energy levels. It is not advisable to use rechargeable batteries in infrequently used devices because they have a higher self-discharge rate. However, if the device requires a large current discharge, rechargeable batteries must be used. It is best for users to choose the appropriate battery for the device based on the manufacturer’s instructions provided in the user manual.
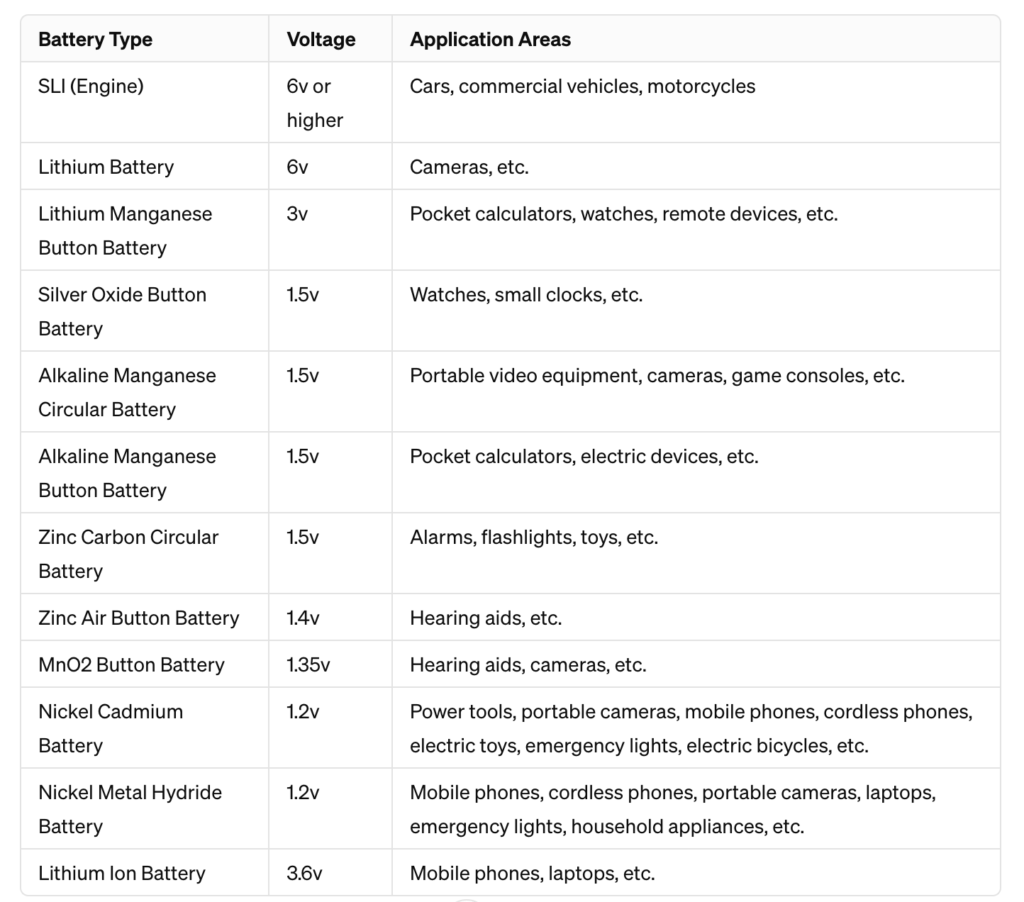
89. What are the voltage and application areas of different types of batteries
90. What types of rechargeable batteries are there? What devices are they suitable for?

91. What types of batteries are used in emergency lights?
Sealed Nickel-Metal Hydride Battery;
Adjustable Valve Lead Acid Battery;
Other types of batteries that meet the corresponding safety and performance standards of IEC 60598 (2000) (Emergency lighting section) can also be used.
92. How long is the lifespan of rechargeable batteries used in cordless phones?
Under normal usage conditions, the lifespan is 2-3 years or longer. The battery needs to be replaced when the following situations occur:
After charging, the talk time becomes shorter each time;
Unclear talk signal, poor reception, and high noise levels;
The cordless phone needs to be closer and closer to the base, indicating a narrowing usage range.
93. What type of batteries can be used for remote control devices?
Remote control devices can only be used by ensuring that the batteries are placed in their fixed positions. Different types of zinc-carbon batteries can be used for different remote control devices. They can be identified by IEC standards and commonly used batteries include AAA, AA, and 9V large batteries. Alkaline batteries are also a good option as they provide twice the working time of zinc-carbon batteries. They can also be identified by IEC standards (LR03, LR6, 6LR61). However, because remote control devices require small currents, zinc-carbon batteries are more cost-effective.
Rechargeable secondary batteries can theoretically be used, but due to their higher self-discharge rates and the need for repeated recharging, they are not very practical for use in remote control devices.
94. What types of battery products are there? And which application areas are they suitable for?
The application areas of nickel-metal hydride batteries include but are not limited to: Electric bicycles, cordless telephones, electric toys, power tools, emergency lights, household appliances, instrumentation, mining lamps, intercoms…
The application areas of lithium-ion batteries include but are not limited to: Electric bicycles, remote-controlled toy cars, mobile phones, laptops, various mobile devices, mini disc players, small cameras, digital cameras, intercoms
Battery and its environment
95. What impact do batteries have on the environment?
Almost all batteries today do not contain mercury, but heavy metals are still necessary components of mercury batteries, rechargeable nickel-cadmium batteries, and lead-acid batteries. If not properly disposed of and in large quantities, these heavy metals can have harmful effects on the environment. There are specialized organizations internationally that recycle manganese dioxide, nickel-cadmium, and lead-acid batteries. For example, the non-profit organization RBRC.
96. How does environmental temperature affect battery performance?
Among all environmental factors, temperature has the greatest impact on the charge and discharge performance of batteries. The electrochemical reactions at the electrode/electrolyte interface are related to the environmental temperature, and this interface is considered the heart of the battery. If the temperature drops, the reaction rate at the electrode also decreases. Assuming the battery voltage remains constant, the discharge current decreases, and the power output of the battery also decreases. Conversely, if the temperature rises, the battery output power increases. Temperature also affects the transport speed of the electrolyte. An increase in temperature accelerates transport, while a decrease slows it down. This also affects the charge and discharge performance of the battery. However, if the temperature is too high, exceeding 45°C, it can disrupt the chemical balance inside the battery, leading to side reactions.
97. What are green and environmentally friendly batteries?
Green and environmentally friendly batteries refer to a class of high-performance, non-polluting batteries that have been in use or under development in recent years. Currently widely used nickel-metal hydride batteries, lithium-ion batteries, and the increasingly popular mercury-free alkaline zinc-manganese primary batteries and rechargeable batteries fall into this category. Additionally, solar cells (photovoltaic cells) that are widely used and convert solar energy into electricity can also be included in this category.
98. What are the "green batteries" currently in use and under research?
New green and environmentally friendly batteries refer to a class of high-performance, non-polluting batteries that have been in use or under development in recent years. Currently widely used lithium-ion batteries, nickel-metal hydride batteries, and the increasingly popular mercury-free alkaline zinc-manganese batteries fall into this category. Additionally, batteries such as lithium or lithium-ion plastic batteries, fuel cells, and electrochemical storage supercapacitors that are under development and research also fall into this category. In addition, solar cells widely used for photovoltaic power generation are also included.
99. Where is the main manifestation of the harmfulness of waste batteries?
Waste batteries listed in the Hazardous Waste Control Catalog that pose significant risks to human health and the ecological environment include mercury-containing batteries, primarily mercury oxide batteries, lead-acid batteries, and cadmium-containing batteries, mainly nickel-cadmium batteries. Improper disposal of these batteries can lead to contamination of soil, water bodies, and the human food chain through consumption of vegetables, fish, and other food items, causing harm to human health.
100. What are the ways in which waste batteries pollute the environment?
The components of these batteries are sealed inside the battery casing during use and do not affect the environment. However, through long-term mechanical wear and corrosion, heavy metals and acids inside can leak out, entering the soil or water sources, and then entering the human food chain through various pathways. The entire process can be summarized as follows: soil or water sources – microorganisms – animals – circulation of dust – crops – food – human body – nervous system – deposition and disease. Heavy metals absorbed by plants and animals from the environment through water sources and food can undergo bioaccumulation through the food chain, accumulating thousands of times higher in higher-level organisms, then entering the human body through food, accumulating in certain organs and causing chronic poisoning.